Potential Hydrogen and Periodic Male Stupidity.
Although they sound similar, they are really two very different things.
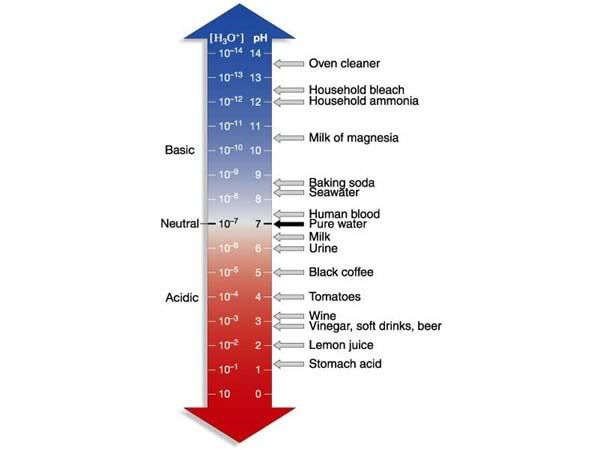
pH is a measure of how many [H+] ions are present in a solution. Its counterpart is the [OH-] ion, or the hydroxide ion. Hydrogen ions are generated when acids are dissolved in water, separating its Hydrogens from the anion. Once inside the solution, the hydrogen ions will react with water molecules to form Hydronium ions (H3O).
If a strong base is dumped into water instead, the [OH-] ions that separate will react with [H+] ions present in the water to form H2O molecules, thereby increasing the pH of the solution.
No comments:
Post a Comment