Theresa is panicking nearby trying to finish her Japanese project. :D So excuse me if this post happens to be filled with nonsensical nonsense.
Chemical reactions are basically the rearrangement of atoms and molecules into new compounds. Heat, bubbles, color changes, property changes, and precipitates (insoluble solild) are all signs of chemical reactions. Chemical reactions happen all around us, even inside our bodies when we breath, eat, and do anything.
Balancing these chemical reactions to have the same amount of atoms on both sides is kind of hard. D: It's kind of like algebra... kinda.
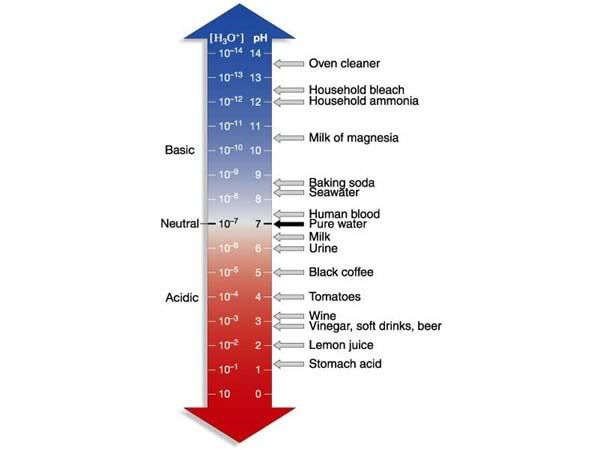
Also I got a 104% on my Solutions and pH test. Yay! :D